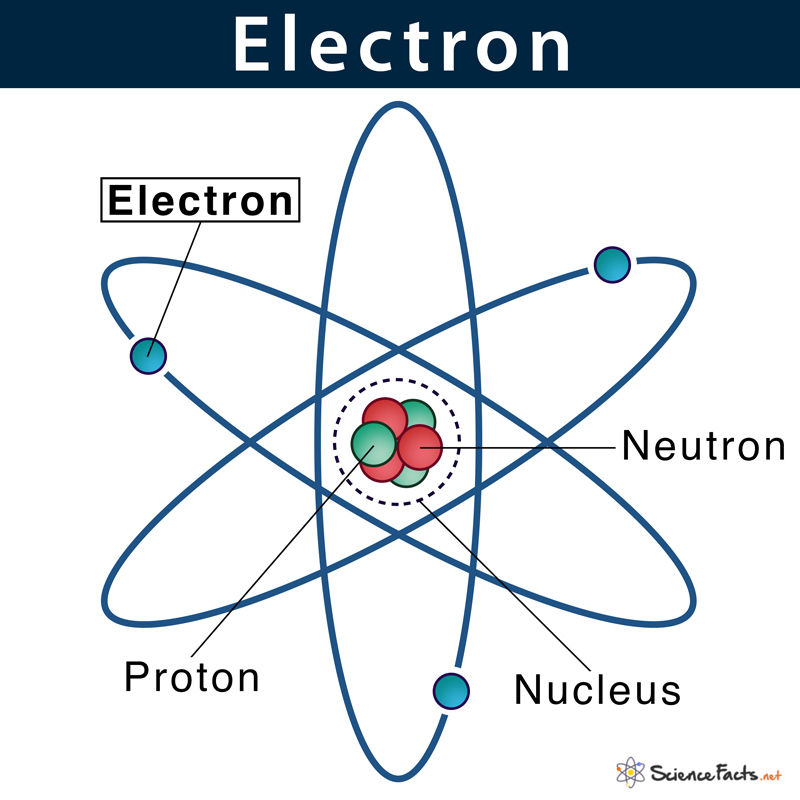
An electron is a subatomic particle that carries a negative
electric charge. It is one of the fundamental particles that make up an atom.
Electrons are found outside the atomic nucleus in regions called electron
shells or energy levels.
Figure 1. Electron
(Cited from https://www.sciencefacts.net/wp-content/uploads/2020/11/Electron-Diagram.jpg)
Key characteristics of electrons include:
- Charge:
Electrons have a negative electric charge (-1 elementary charge). This
charge is equal in magnitude but opposite in sign to the positive charge
carried by protons.
- Mass:
Electrons have a much smaller mass compared to protons and neutrons. The
mass of an electron is approximately 1/1836th of the mass of a proton.
- Behavior:
Electrons exhibit properties of both particles and waves, known as
wave-particle duality. They can behave as discrete particles and also
display wave-like characteristics, such as interference and diffraction.
- Energy
Levels: Electrons occupy specific energy levels around an atomic nucleus.
These energy levels, also known as electron shells or orbitals, are
quantized, meaning they can only have certain discrete values.
- Electron
Cloud: Electrons do not follow a fixed path or orbit around the nucleus.
Instead, they are described by a probability distribution known as the
electron cloud or electron orbital. The electron cloud represents the
region where an electron is most likely to be found.
- Charge
and Matter Interactions: Electrons play a crucial role in chemical
reactions and the formation of chemical bonds. Their interactions with
other atoms determine the electrical and chemical properties of
substances.
Electrons are fundamental to the understanding of
electricity, magnetism, and the behavior of matter at the atomic and subatomic
level. They are also essential for the functioning of electronic devices and
play a vital role in various fields of science, including physics, chemistry,
and materials science.
External resources for learning: